Which of the Subatomic Particles Contributes Almost No Weight
Electrons at various energy levels. Advertisement New questions in Biology.

Ppt Chapter 1 Powerpoint Presentation Free Download Id 5376958
The electrons will contribute almost no weight to the atom.
. Electrons at various energy levels. Protons in the electron shells. Which of the subatomic particles contributes almost no weight to an atoma.
What subatomic particle or particles contributes the most to the mass of the atom. Click to see full answer In respect to this which subatomic particles contribute to an atoms mass number but not atomic number. - a protons c protons and electrons b neutrons - d protons and neutrons 8.
How many shells an atom has. Electrons in the nucleusc. How many electrons are in the outer shell.
The electrons will contribute almost no weight to the atom. Isotopes of the same element differ from each other only by the number of neutrons. However the most fundamental subatomic particles are electrons protons and neutrons.
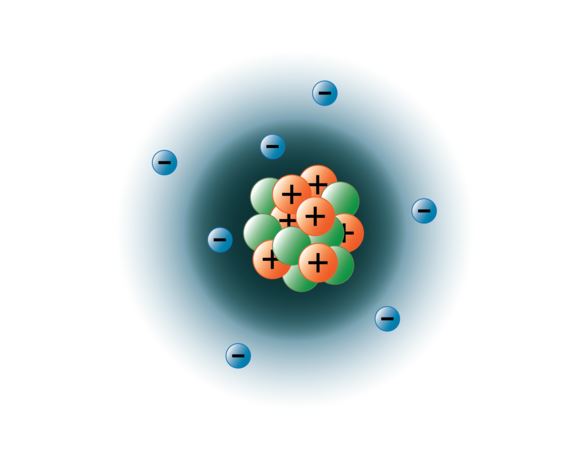
Subatomic particles include electrons negatively charged nearly massless particles that account for much of the atoms bulk that include the stronger building blocks of the atoms compact yet very dense nucleus the protons that are positively charged and the strong neutrons that are electrically neutral. Protons in the electron shells b. As stated before there are more than 200 subatomic particles that exist in nature.
Protons in the electron shells. However the mass of them are the same and the mouth of an electron is significantly less than the mass of a neutrons. The atomic number tells you the.
Electrons in the nucleusc. The atomic number symbol. Problem Which of the subatomic particles contributes almost no weight to an atom.
The electron is the lightest particle in the atom. Answer is going to be D. Electrons at various energy levels.
The mass of an electron is only about 12000 the mass of a proton or neutron so electrons contribute virtually nothing to the total mass of an atom. Which of the subatomic particles contributes almost no weight to an atom. Neutrons in the nucleus.
An atom that has two electrons in the outer shell such as magnesium would most likely. Protons in the electron shellsb. What subatomic particle contributes almost no weight to an atom.
Electrons in the nucleus c. Electrons in the nucleus. Neutrons in the nucleusd.
Lose two electrons and become a. Protons neutrons and electrons are the 3 what. The atomic number symbol and mass.
An atom contain subatomic particles. Electrons revolve in shells and contribute almost nothing to the total mass of an atom. Neutrons in the nucleus.
Protons in the electron shells b. Electrons at various energy levels. Which of the subatomic particles contributes almost no weight to an atom.
However the mass of them are the same and the mouth of an electron is significantly less than the mass of a neutrons. Protons in the electron shells b. Atomic number - Number of protons housed in.
Carbon 14 is an unstable isotope of carbon that decays very slowly. Which of the subatomic particles contributes almost no weight to an atom. Which of the subatomic particles contributes almost no weight to an atom.
Which of the subatomic particles contributes almost no weight to an atoma. So theres gonna be a higher energy level for this electron there would be for the inner one. Connect Online for Biology 10th Edition Edit edition Solutions for Chapter 2 Problem 1TY.
Which subatomic particles contribute to the atomic mass for any given element. So theres gonna be a higher energy level for this electron there would be for the inner one. Electrons in the nucleus.
Which of the subatomic particles contributes almost no weight to an atom. Electrons in the nucleus c. Answer is going to be D.
Electrons at various energy levels. Protons in the electron shellsb. Properties of Subatomic Particles and their Charges.
Neutrons in the nucleusd. View Notes - IMG_20210311_152922_755jpg from BIOL 82 at San Jacinto College. Although similar in mass protons are positively charged while neutrons have no charge.
Therefore the number of neutrons in an atom contributes significantly to its mass but not to its chargeElectrons are much smaller in mass. LearnSmart Online for Biology 10th Edition Edit edition Solutions for Chapter 2 Problem 1TY. Biology 10th Edition Textbook Solutions.
Electrons in the nucleusc. These particles are protons and neutrons. What is a subatomic particles with almost no mass.
View the primary ISBN for. What subatomic particle is so small that it has no mass it is not included. When an electron loses its electrons it turns into a ___ charged ion.
Compared to the common stable carbon 12 isotope carbon 14 has two additional a electrons b neutrons c. Protons in the electron shellsb. Which of the subatomic particles contributes almost no weight to an atom.
Which of the subatomic particles contributes almost no weightto an atoma.

Solved Which Of The Subatomic Particles Contributes Almost No Weight To An Atom A Protons In The Electron Shells B Electrons In The Nucleus C Neutrons In The Nucleus D Electrons At Various

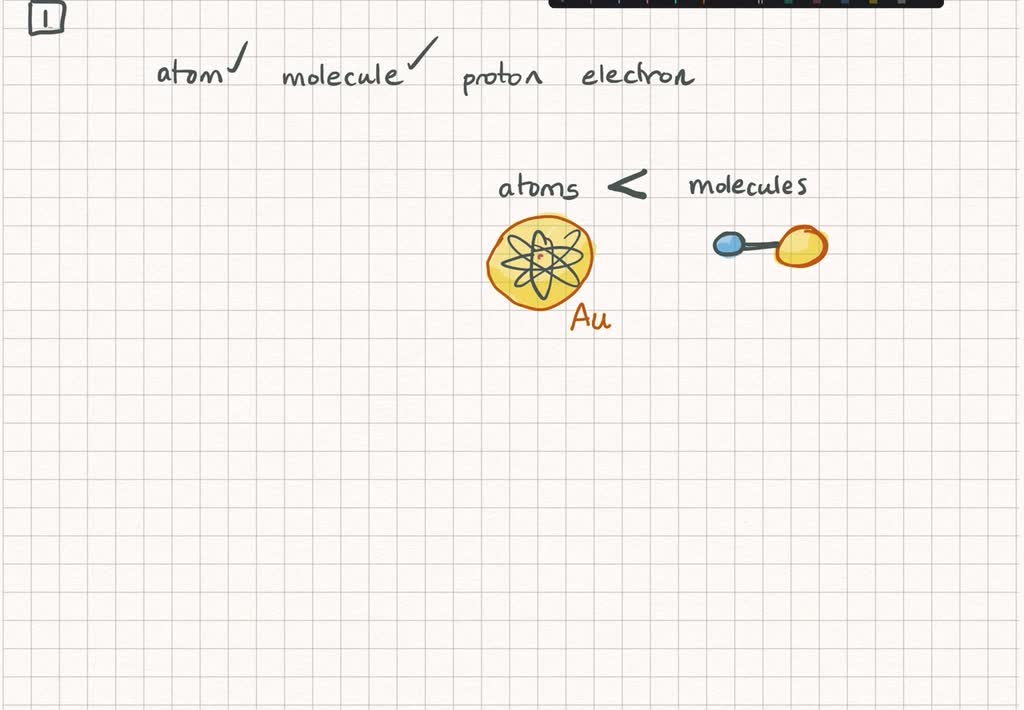
Solved Choose The List That Goes From The Lightest To The Heaviest A Proton Atom Molecule Electron B Atom Proton Molecule Electron C Electron Proton Atom Molecule D Atom Electron Proton Molecule E
No comments for "Which of the Subatomic Particles Contributes Almost No Weight"
Post a Comment